The Hazari group has had a long interest in the design of transition metal catalysts for pharmaceutical applications. One of our most significant discoveries was the development of a family of highly active Pd precatalysts for cross-coupling (Figure 1). These precatalysts are now commercially available on a kilogram scale from Umicore.
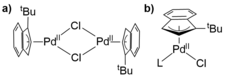
Figure 1. Our commercially available a) unligated and b) ligated Pd precatalysts.
Due to sustainability concerns our focus shifted from Pd to Ni and we spent several years understanding the mechanism of Ni-catalyzed cross-coupling reactions. Currently, our work focusses on cross-electrophile coupling (XEC) reactions, in which two electrophiles are coupled by a transition metal catalyst, typically Ni, in the presence of an electron source. These reactions are particularly effective for the formation of C(sp2)–C(sp3) and C(sp3)–C(sp3) bonds, which are difficult to form using other synthetic methods, such as cross-coupling. However, currently the key elementary steps in XEC reactions are not always well understood. Our goal is to deconvolute XEC reaction mechanisms and design new synthetic methods by investigating two critical areas in XEC: the transition metal catalyst and the electron source.
In XEC reactions, it is important to control the rate of activation of both substrates in order to avoid side reactions, such as homocoupling. However, this is difficult to achieve when both electrophilic substrates are activated by the same metal catalyst. We recently developed a dual-catalytic XEC method for aryl and alkyl halides employing Ni and Co complexes as cocatalysts and a homogeneous reductant, TDAE, as the electron source (Figure 2). Specifically, the Ni catalyst activates the aryl halide while the Co catalyst engages in a different catalytic cycle to activate the alkyl halide. The use of a homogeneous reductant is crucial as it prevents the Ni catalyst from reacting with the alkyl substrate. The dual catalytic system significantly expands the substrate scope of the coupling between aryl and alkyl halides. We are currently investigating new dual catalyzed reactions to couple aryl halides with more challenging alkyl halides that are incompatible with the Ni/Co system.
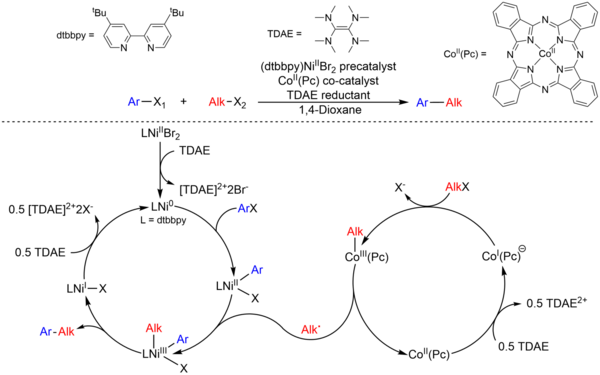
Figure 2. Proposed mechanism for the XEC of aryl and alkyl halides using Ni/Co dual catalytic system with TDAE.
Unlike the commonly proposed PdII/0 cycles for cross-coupling reactions, most of the mechanistic proposals of XEC reactions include Ni species with multiple oxidation states ranging from 0 to +3. In particular, NiI species have been proposed as important intermediates in various types of XEC reactions. To better understand their role in catalysis, we have synthesized NiI complexes bearing different bidentate nitrogen ligands (Figure 3). Characterization and reactivity studies on those complexes show that the steric and electronic properties of the supporting ligand can have dramatic effects on the speciation and reactivity of the NiI complex. As a result, we are currently working on developing structure-activity relationships using NiI complexes, will ultimately guide catalyst design and result in new and improved XEC reactions.
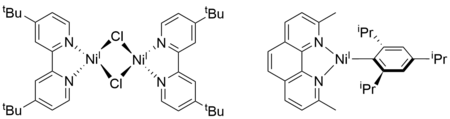
Figure 3. Examples of bidentate nitrogen-ligated NiI complexes.
Heterogeneous reductants, such as Zn0 or Mn0 powder, are often used as the electron source in XEC reactions due to their cheap price and air stability. However, the use of heterogeneous reductants can introduce engineering problems in large scale process chemistry and in medicinal chemistry that frequently uses high throughput-equipment for optimization. Moreover, it is difficult to conduct mechanistic studies with heterogeneous reductants due to irreproducible kinetics that arise from mass transfer limitations. While homogeneous reductants, such as TDAE, can solve some of these problems, they are rarely used in XEC due to their high price and poor air stability. We developed four new homogeneous reductants based on the tetraaminoethylene scaffold found in TDAE. The reductants span nearly ~500 mV in reduction potential and are considerably more air stable than TDAE (Figure 4). In addition, we have utilized the weakest reductant, TME, for a XEC reaction between aryl iodides and benzylic Katritzky salts that require controlled radical generation from a weak reductant. We are interested in developing more homogeneous reductants that would span a larger range of reduction potentials for different synthetic methods.
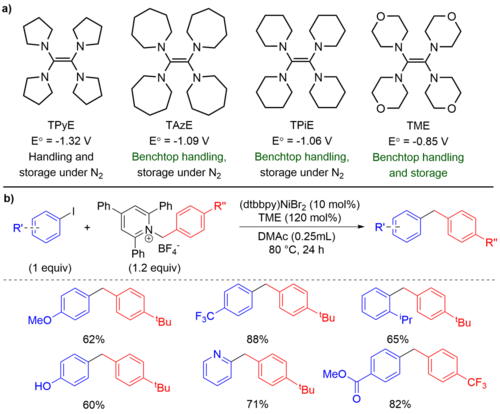
Figure 4. a) Newly synthesized homogeneous reductants with variable E° and enhanced air stability in comparison to TDAE. Potentials are reported relative to ferrocene (Fc) in DMF. b) XEC reactions between aryl iodides and benzylic Katritzky salts facilitated by TME.