The rising concentration of CO2 in the atmosphere and the depletion of our fossil fuel reserves makes it desirable to produce fuels using CO2 as a feedstock. Systems that can convert CO2 into fuels using sunlight are particularly desirable given that solar energy is our single largest energy source. The Hazari group is a member of CHASE (The Center for Hybrid Approaches in Solar Energy to Liquid Fuels), which is a DOE funded collaborative hub that brings together scientists from UNC Chapel Hill, Yale, Penn, Brookhaven National Laboratory, Emory, and North Carolina State University. CHASE’s mission is to generate liquid fuels from CO2, N2, or H2O using sunlight. The CHASE approach is to combine the light-absorbing properties of semiconductors with the selective fuel-producing reactivity of molecular catalysts to develop molecule/material hybrid photoelectrodes (Figure 1). The fabrication of hybrid photoelectrodes requires the preparation of molecular catalysts with surface attachment groups. Accordingly, the Hazari group’s role in CHASE is to prepare molecular catalysts for CO2 reduction with modified ancillary ligands that allow surface attachment to a light-absorbing semiconductor surface, such as Silicon. We have developed synthetic routes to incorporate functional groups for surface attachment, such as silatrane, alkene, alkyne, and pyrene moieties into existing molecular CO2 reduction catalysts. Beyond the synthesis of molecular catalysts, the Hazari group also works in the development of Silicon-based photoelectrodes that incorporate catalysts that reduce CO2 to CO or formate. These processes are the initial reactions in a series of catalytic cascades, currently under development by other CHASE researchers, that will ultimately upgrade these products to methanol, ethanol, and higher-mass alcohols. The performance of our hybrid photoelectrodes is tested in our own state-of-the-art photoelectrochemical station which includes a Solar Simulator, Electrochemical Analyzer, and Gas Chromatograph set up for in line quantification of gaseous products. Liquid products are analyzed using instrumentation in the department of Chemistry at Yale. The Hazari lab is also interested in understanding how the microenvironment impacts the redox and CO2-reduction properties of molecular catalysts. In collaboration with the group of Prof. M. Zhong (Yale Dept of Chemical Eng.) and S. Hammes-Schiffer (Yale Dept of Chemistry) we are exploring how Lewis Acids impact the electrochemical behavior of a family of cobalt porphyrins and how these changes may translate into improved activity, selectivity, and/or overpotential for CO2 reduction. Finally, the Hazari group is involved in multiple collaborations with other CHASE groups with expertise in advanced spectroscopic and surface characterization techniques to understand how molecular catalysts bind to a surface.
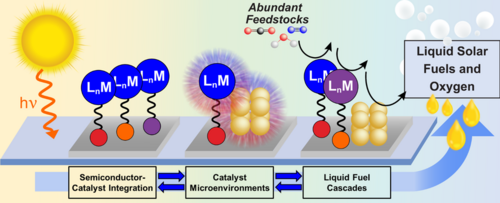
Figure 1. Schematic representation of the CHASE approach to develop hybrid photoelectrodes that convert abundant feedstocks to liquid fuels.