Carbon dioxide is an attractive target as a C1 chemical feedstock due to its low cost, non-toxic nature, and high abundance. Therefore, developing catalytic methods to convert CO2, a natural waste product, into more valuable chemicals such as methanol, formic acid, or carboxylic acids will present both economical and environmental benefits to society. While there are several chemicals which are industrially derived from CO2, such as urea and carbamates, this represents a small fraction of available CO2. The limiting factor of fully realizing the potential of CO2 as a feedstock is due to its inherently high kinetic and thermodynamic stability, making its chemical conversion a challenge. The use of homogeneous transition metal catalysts is a promising strategy for the conversion of CO2 to valued-added products. One of the most ubiquitous reaction pathways in this facet involves the insertion of CO2 into M–E bonds (X = H, OR, CR3). In particular, the insertion of CO2 into late transition metal M–E bonds exploits the relative weakness of the M–O bond, which is formed, making it an attractive target for further functionalization. We are interested in performing fundamental mechanistic and kinetic studies on CO2 insertion reactions into late transition metal-element bonds and gaining insight into how we can modify factors such as ligand donors, solvent, and Lewis acid additives to assist the design of future CO2 utilization catalysts.
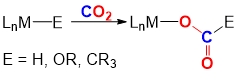
Figure 1. CO2 insertion into a M–E bond.